Join devRant
Do all the things like
++ or -- rants, post your own rants, comment on others' rants and build your customized dev avatar
Sign Up
Pipeless API

From the creators of devRant, Pipeless lets you power real-time personalized recommendations and activity feeds using a simple API
Learn More
Related Rants

 Found on Reddit, with @vanz
Found on Reddit, with @vanz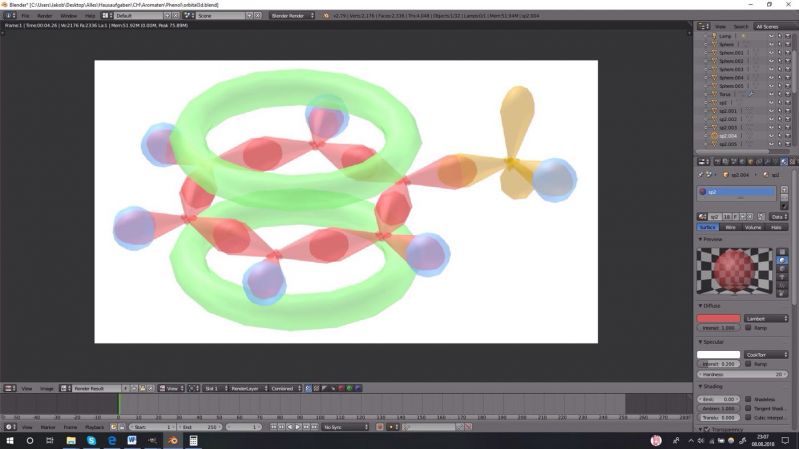 Homework:
Create a fact file and orbital molecule to the substance I've adviced you.
Me:
- 1min ctrl+c ctrl+v...
Homework:
Create a fact file and orbital molecule to the substance I've adviced you.
Me:
- 1min ctrl+c ctrl+v...
Warning - Not IT related.
Long ago, I had a chemist professor who told us a story. She worked in a laboratory where they have studied cristal formations, so basically made a liquid highly capable to form cristals, and they watched them forming, doing tests and so on. In the meantime new building of the campus opened and they had to move the lab to the new location, which was a fourth floor of newly made building. Few of them started to work there even before they moved the old materials and equipment and they started few cristalisation studies, the interesting part is that the cristals didn't formed. She said that at the end they had many cups with prepared liquid and apsolutely no cristals for weeks, but one day the lead researcher arrived with the old, already formed cristals, from the old lab, and toon those inside of the room with prepared cups all the cristals started to form at the same time. After telling us a story she asked us not to tell this to anyone because the science currently doesn't accept this phenomenon and we will be demonised and looked fools it the scientific community.
This story made a hole in my brain...
It was like 10 years ago, and as a problem solver I still have sometimes some weird ideas about it, and strange explanations comming from nothing, and without any deep understanding of quantum physics or even cristalisation. :D
rant
something else
not it
chemistry